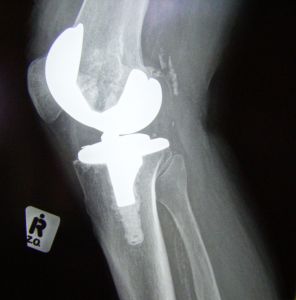
Johnson & Johnson is currently facing more than 11,500 lawsuits throughout the United States. Our Boston defective hip attorneys know that Johnson & Johnson is facing these lawsuits as a result of DePuy hip implant products. The DePuy products are manufactured by a Johnson & Johnson subsidiary and, unfortunately, have a high failure rate and can cause patients to experience serious complications. 
Johnson & Johnson is reportedly considering making a large settlement offer to resolve as many of the lawsuits as possible. According to Bloomberg.com, the company hopes to resolve cases arising from DePuy hip replacement devices by early next year.
Johnson & Johnson Settlement Would Be the Largest To-Date Related to Hip Implant Defects
DePuy, a unit of Johnson & Johnson, is facing massive potential legal liability as a result of its defective hip implant products. In 2010, the company was forced to recall 93,000 implants, an estimated 37,000 of which had been implanted in patients in the United States. The recall was prompted by the fact that 12 percent of all of the DePuy hip implant and replacement devices had failed within five years (a much higher failure rate than for other hip replacement solutions).
Unfortunately, the failure rate of the DePuy products doesn’t seem to be declining but instead appears to be getting worse in recent years. Further, the number of lawsuits has also been on the rise, with patients suing to recover monetary compensation for pain and for the cost of replacement surgeries when the DePuy hip products fail.
There is some good news for victims, however. In the first lawsuit arising from DePuy hip implant devices, the court found in the plaintiff’s favor and Johnson & Johnson lost an $8.3 million verdict. While the second lawsuit saw Johnson & Johnson prevail, the company still faces many potential viable claims.
Johnson & Johnson wants to resolve cases by compensating those who have been harmed, rather than by going to trial. The company is reportedly considering payout out a settlement of more than $300,000 per case. This would result in a total settlement in excess of $3 billion, which is 50 percent higher than the settlement offer proposed in recent negotiations.
If the $3 billion settlement were to occur, this would be the highest amount of money paid to settle a hip implant claim, topping the prior record, which was set when a company called Sulzer agreed to pay $1 billion in 2001 to resolve lawsuits against it.
Whether Johnson & Johnson is actually able to resolve its cases through settlement will depend upon a number of factors, including how many plaintiffs are willing to accept the proposed settlement and how several product-liability trials go between September and January. The first of the seven pending claims that could have an impact on settlement is scheduled to begin on September 9 in Cleveland. The judge in Cleveland is overseeing approximately 8,000 federal cases that were consolidated.
In the meantime, Johnson & Johnson has already spent approximately $993 million on informing patients of the recall of the defective DePuy hip implants. The company also continues to provide a reimbursement program to patients who have DePuy hip implants and who require recall-related testing or treatment necessitated by the recall of the dangerous hip implant product. The high costs and large potential settlement should serve as a reminder to medical device companies of the importance of ensuring that medical products are safe.
Continue reading

 Boston Personal Injury Attorney Blog
Boston Personal Injury Attorney Blog